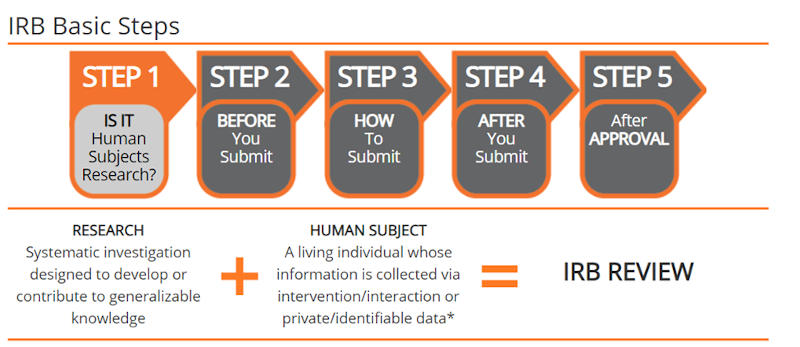
All CCC employees (faculty/staff/administrators) and students conducting human subjects research, as defined by the Department of Health and Human Services (DHHS) and the Federal Drug Administration (FDA), need to submit required materials to the Office for Research Protections (ORP) for review before beginning any research activity
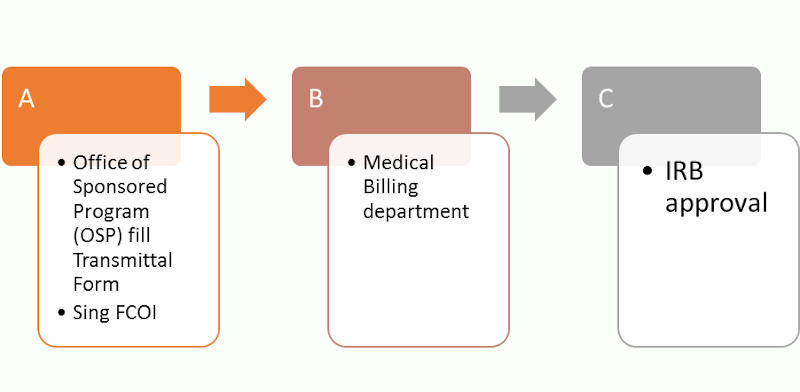
For Industry Sponsor Study